header
News
Decoding the Lupus Gene: Professor Kwangwoo Kim of the Department of Biology Unlocks the Origin of Lupus
- WRITER 학무부총장실
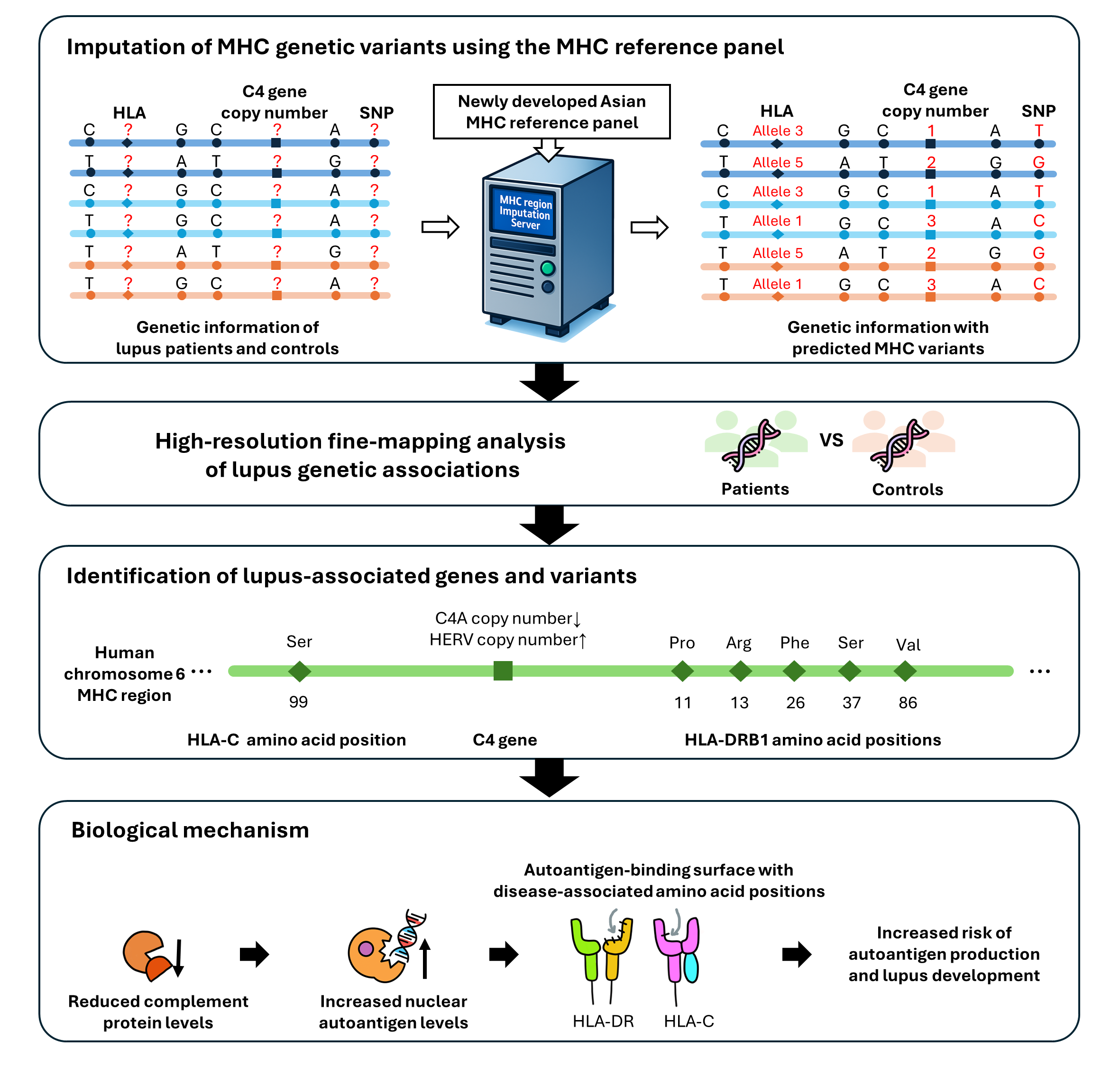
Professor Kwangwoo Kim's Research Team at the Department of Biology develops MHC genetic variation reference panel
Identification of Lupus-causing genetic variants through precise genome analysis of approximately 70,000 individuals
Systemic lupus erythematosus (SLE), commonly known as lupus, is a well-known autoimmune disease in which the immune system mistakes healthy cells for foreign invaders and attacks them, causing inflammation and tissue damage in major organs across the body. While complex factors, including genetics, environment, and sex hormones, contribute to risk, the exact pathogenesis of lupus has remained unclear.
In response to this challenge, Professor Kwangwoo Kim's research team at the Department of Biology developed a customized reference panel tailored for the Koreans, capable of high-resolution analysis of genetic information in the Major Histocompatibility Complex (MHC) region of human DNA. Using this panel, the team analyzed genome data from approximately 70,000 individuals and identified key genetic variants closely linked to the development of lupus.
MHC region: a key DNA area in understanding lupus genetics
The research focused on the MHC region of chromosome six in human DNA, which is densely packed with a high concentration of immune-related genes. Previously, large-scale, precise analyses on this area were limited due to the complex genetic structure. However, the newly developed tool can simultaneously predict HLA gene clusters and C4 gene mutations, significantly improving analytical accuracy.
Using this comprehensive reference panel, the research team conducted a precise analysis of genome data from approximately 70,000 Korean individuals, including both lupus patients and healthy controls. The results revealed that individuals with deficiencies in the C4 gene had a roughly 1.4-fold higher risk of developing lupus compared to those without such deficiencies. Conversely, each additional copy of the C4 gene was associated with a roughly 31% reduction in the risk of lupus. Furthermore, this study also found that certain amino acid mutations in the HLA gene can alter how the HLA protein binds to antigens, inducing structural changes that can cause autoantigens to be misidentified as foreign threats. In particular, when the number of C4 gene copies is reduced, or when an abnormally long untranslated sequence is inserted, the production of complement protein production decreases, which in turn increases the risk of lupus by heightening immune system imbalance. These results demonstrate that the abnormal immune responses observed in lupus patients are closely and inherently linked to genetic risk factors.
Possibility of early diagnosis and personalized treatment; laying the foundation for precision medicine
This study elucidated the genetic pathogenesis of lupus, revealing how abnormal immune response is closely tied to genetic variation. The identification of such genotypes opens the door to risk prediction, early disease detection, and the creation of treatment frameworks tailored to an individual’s genetic profile. The presence of these specific genotypes provides a concrete basis for developing precision medicine therapies designed to counteract the precise molecular mechanism at work.
The reference panel developed by the research team will be made available through the National Institute of Health's CODA system and is anticipated to see broad application in genetic research spanning autoimmune, infectious, and inflammatory diseases. It holds particular significance as a public infrastructure resource, enabling large-scale and highly precise analysis of the MHC region, an area of genomic study that has long been technically challenging with existing methodologies.
This study was conducted jointly by Professor Kwangwoo Kim and his team at the Department of Biology, Hanyang University, the National Institute of Health, and the Ulsan National Institute of Scient and Technology (UNIST), with support from the National Research Foundation of Korea. The findings were published on July 5, 2025, in Annals of the Rheumatic Diseases (IF 20.6), a leading international journal in rheumatology, under the title, “Development of an MHC imputation panel highlights independent contributions of HLA amino acid residues and C4 copy number variations to SLE risk.”
