header
News
Opening Up the Possibility of Ginseng-derived Nanomaterials to Treat Infectious Diseases
- WRITER 학무부총장실
A joint research team, headed by Professors Yeon-Ju Kim of the College of Life Sciences and Ik Hyun Cho of the College of Korean Medicine, demonstrated the efficacy of natural product-based nanomaterials derived from ginseng in suppressing COVID-19 infection and pulmonary inflammation
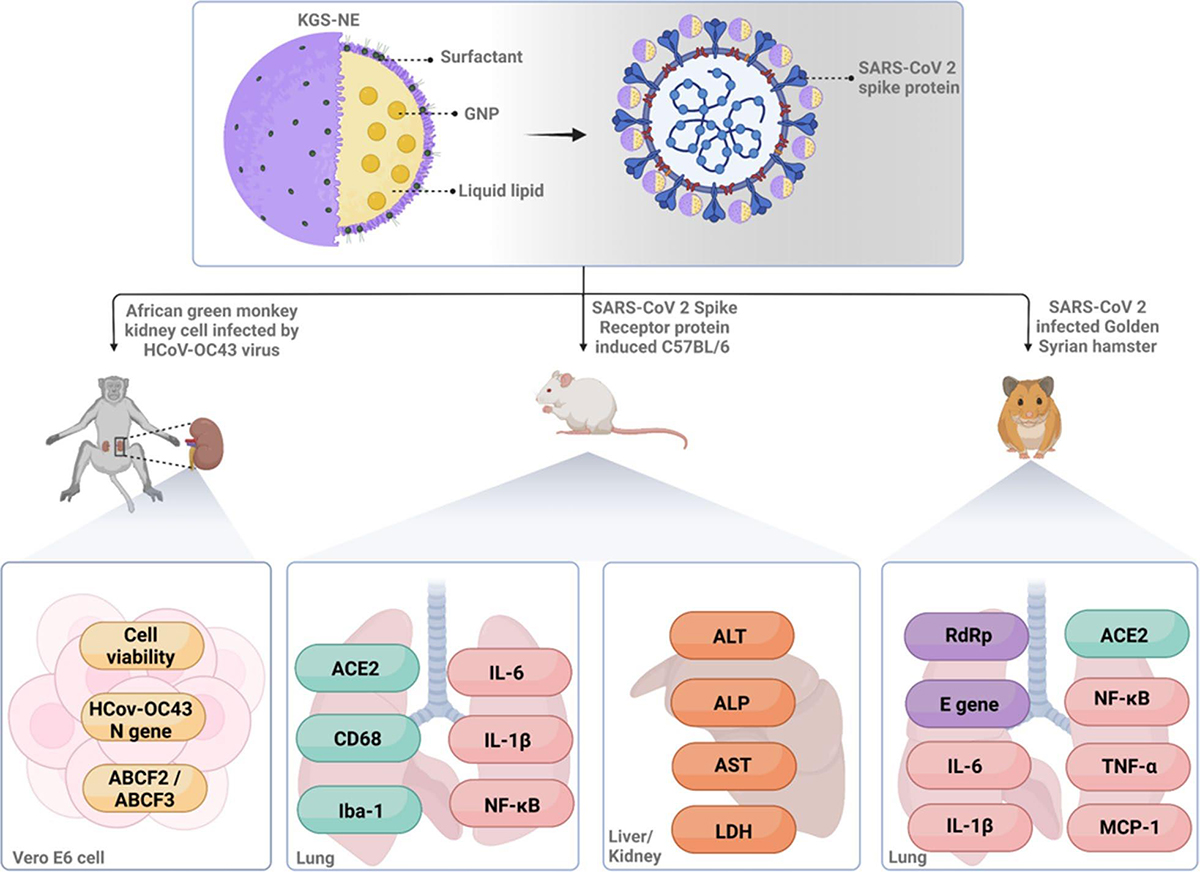
A joint research team led by Professors Yeon-Ju Kim of the College of Life Sciences and Ik Hyun Cho of the College of Korean Medicine successfully demonstrated that ginseng-derived natural nanomaterials can suppress COVID-19 infection and alleviate lung inflammation. The research findings were published on January 15, 2025, in Journal of Nanobiotechnology (IF: 10.6) under the title, “Lipid-encapsulated gold nanoparticles: an advanced strategy for attenuating the inflammatory response in SARS-CoV-2 infection.” The research team developed an oral Korean ginseng nanoemulsion (KGS-NE), combining ginseng seed oil (GSO) and gold nanoparticles (GNP), and demonstrated both antiviral and immunoregulatory effects of KSG-NE. This breakthrough is garnering significant attention within the academic community as a promising example of integrating natural products with nanotechnology for the treatment of infectious diseases.
Among existing COVID-19 treatments, several drugs have raised concerns due to side effects and adverse interactions. In response, the research team has proposed an alternative strategy that integrates traditional herbal medicines with nanotechnology. The oral nanoemulsion KGS-NE developed by the team demonstrates high systemic absorption rate and enables stable drug delivery. Its excellent biocompatibility and storage stability that complement the limitations of existing nanomaterials have received much attention as a promising alternative. Notably, KGS-NE remains stable for over six months and resists degradation in the gastrointestinal environment, further reinforcing its therapeutic viability.

Immune response control mechanism also confirmed; expected to suppress cytokine storm
The research team confirmed the therapeutic efficacy of KGS-NE using in vivo models of SARS-CoV-2 infection, including Syrian hamsters and C57BL/6 mice. Oral administration of KGS-NE suppressed viral infection and significantly reduced viral replication. In animal models exhibiting acute pulmonary inflammation following infection, KGS-NE attenuated the level of lung tissue damage, while controlling weight loss and improving the survival rate.
Molecular-level analysis showed that KGS-NE acts on key inflammatory signaling pathways, effectively modulating the expression of pro-inflammatory cytokines such as NF-κB, IL-6, IL-1β, and TNF-α. This regulatory mechanism on the immune response suggests that KSG-NE mitigates lung tissue damage by counteracting the cytokine storm, a major pathological driver of severe COVID-19 symptoms.
Through RNA sequencing, the research team analyzed gene expression and confirmed that KGS-NE suppresses ABCF2 and ABCF3—genes implicated in immune response modulation—thereby simultaneously inhibiting viral activity and activating host defense mechanisms. These findings suggest that KGS-NE functions not merely as a direct antiviral agent but as a comprehensive therapeutic strategy that mitigates tissue damage through precise immune regulation.
Potential for combination therapy with existing treatments, and a new direction in infectious disease response
Through this study, the research team experimentally demonstrated the therapeutic potential of natural product-based nanotechnology in the context of infection disease treatment. In particular, KGS-NE shows promise as a new therapeutic agent capable of synergizing with existing antiviral drugs such as Molnupiravir and Paxlovid. Building on these findings, the team plans to conduct follow-up clinical studies to further evaluate the safety, efficacy, and translational viability of KGS-NE as a practical treatment option.
This study represents a new paradigm in infectious disease treatment through the convergence of biotechnology and Korean medicine. It demonstrated the potential to transform traditional herbal medicine into modern nanotherapeutics. Professor Kim explained, “This work provides experimental validation for the development of natural product-based antiviral treatments using nanotechnology. It offers an alternative therapeutic approach distinct from conventional synthetic antiviral drugs.”
Professor Cho said, “Grounded in the immune-regulatory principles of Korean medicine, this research has confirmed that the convergence of natural products and nanotechnology can contribute meaningfully to new drug development. We plan to expand its application not only for COVID-19, but also for a broader range of viral infections and immune-mediated diseases through future clinical studies.”
Dr. Sanjeevram Dhandapani, co-first author and researcher at the Department of Convergent Biotechnology and Advanced Materials Science, said, “Building on the results of this study, I plan to pursue my research on organoid-based infectious disease models at Duke University and continue exploring avenues for therapeutic development.” Yujeong Ha, Ph.D. candidate at the Department of Convergence Medical Science and also co-first author, added, “This was a meaningful opportunity to confirm the potential of integrating natural products with nanotechnology. I would like to continue to contribute to the development of therapeutic strategies.”
This study was supported by Mid-career Researcher Support Program of the National Research Foundation of Korea.