header
News
Professor Jin-Woo Bae’s Team Publishes Literature Review on Advances in Gut Microbiome-Based Treatments
- WRITER 학무부총장실
Professor Jin-Woo Bae’s research team from the Department of Biology has published a comprehensive review examining the efficacy and limitations of two prominent gut microbiome therapies: fecal microbiota transplantation (FMT) and live biotherapeutic products (LBPs)
A research team led by Professor Jin-Woo Bae at the Department of Biology has published a comprehensive review examining two leading gut microbiome therapies that are reshaping treatment approaches for various diseases. The study provides a critical analysis of fecal microbiota transplantation (FMT) and live biotherapeutic products (LBPs), highlighting the latest research trends and key challenges in the development of microbiome-based treatments. The research results were published online in October 2024 in Gut Microbes (Impact Factor: 12.2) under the title, “Gut microbiome therapy: fecal microbiota transplantation vs live biotherapeutic products.”
Reviewing the Last Five Years: Challenges and Future Directions in Microbiome-Based Treatments
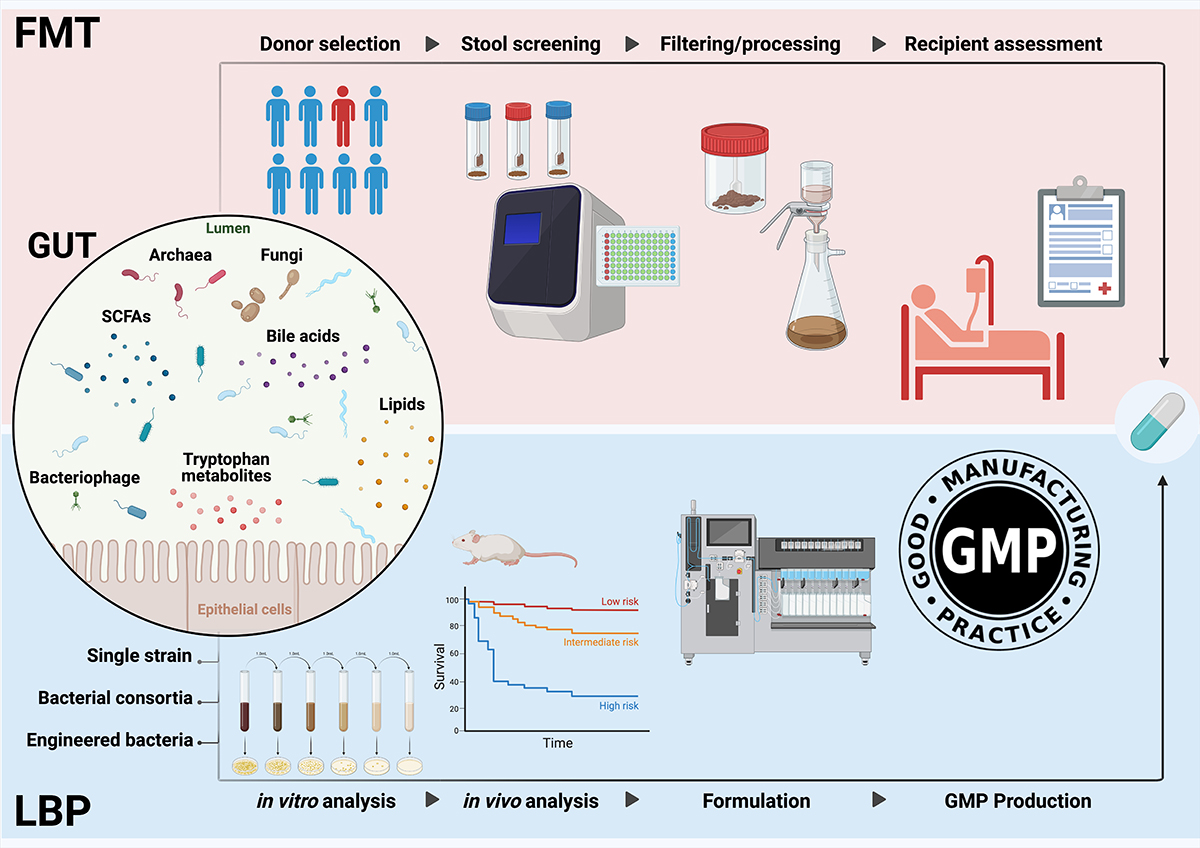
The research team conducted an in-depth review of literature published over the past five years, analyzing numerous studies to provide a comprehensive overview of the latest developments in microbiome-based therapies. The review focused on diseases where microbiome-based treatments have been actively applied, spanning a wide range of conditions, including gastrointestinal disorders, metabolic diseases, and cancer. By comparing clinical applications, therapeutic outcomes, and limitations, the team also identified future research directions to address current gaps and further advance the field.
Gut microbes refer to the collection of bacteria, viruses, fungi, and other microorganisms that reside in the digestive system. Research has shown that the gut microbiome influences not only gastrointestinal metabolism and immune functions but also affects diseases in other organs such as the liver and brain. The ‘gut-brain axis’ and ‘gut-liver axis’ are terms that have emerged to describe the bidirectional communication between the gut and various organs, emphasizing the growing understanding of the gut’s impact on overall health.
An imbalance in the gut microbiome has been linked to several conditions, including Clostridioides difficile infection, inflammatory bowel disease, various metabolic disorders, neurological diseases, and cancer. This makes therapies aimed at restoring gut microbiome balance a promising next-generation treatment option. FMT and LBPs are two such therapies currently being explored.
Fecal microbiota transplantation (FMT) involves transferring fecal matter from a healthy donor to a patient’s gut to restore the microbiome’s balance. It has shown considerable effectiveness in treating recurrent Clostridioides difficile infections and has potential for use in certain inflammatory bowel diseases and metabolic disorders. Live biotherapeutic products (LBPs), on the other hand, provide a more targeted approach by using specific strains of bacteria or bacterial mixtures tailored to treat particular diseases. Unlike FMT, LBPs are typically designed for specific conditions and are often in clinical trials to assess their efficacy and safety across a range of diseases.
Professor Bae’s team provided a detailed overview of the research trends and current status of both therapies, highlighting the unresolved challenges that researchers continue to face. For FMT, key challenges include selecting appropriate donors and managing the risk of potential infections. In the case of LBPs, a significant issue is the lack of interaction with other beneficial microbes or metabolites in the body. Both therapies share common challenges, such as the need for standardization and long-term stability assessment.
Professor Bae explained the significance of this study, saying: “While previous research has focused on the efficacy and application of fecal microbiota transplantation and live biotherapeutic products, there has been a lack of comparative analysis between the two. This study fills that gap by comparing the clinical applications of both therapies and providing a comprehensive analysis of their respective efficacy and limitations.”
Student Do-Yeon Kim (Master’s in Biology), the first author of the study, reflected on her experience: “Writing my first paper was challenging. Organizing the research and creating a clear structure was difficult, especially when reviewing such a large boy of literature. I spent considerable time ensuring consistency between sources and refining how to present complex ideas within the paper’s flow. What I learned was that it’s not just about compiling data—it’s about critically analyzing it and making clear connections to previous studies. It wasn’t easy, but I’m confident this experience has laid a strong foundation for my future research.”
This study was conducted as part of a research project sponsored by the Ministry of Food and Drug Safety, aimed at standardizing the efficacy of gut microbiome-based therapies.
For more information on the study: https://www.tandfonline.com/doi/full/10.1080/19490976.2024.2412376